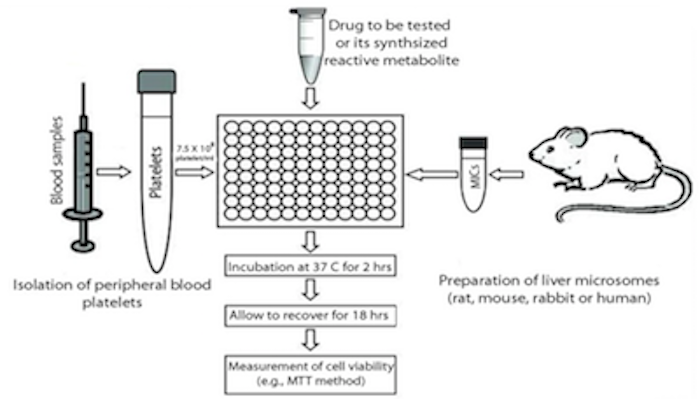
The in vitro Platelet Toxicity Assay
Recent research in our laboratory focused on developing and validating the use of peripheral blood platelets (PBPs) as a surrogate cell model for in vitro toxicity testing. Due to their small size and low density, PBPs are readily collectable from blood using differential centrifugation. In addition to blood homeostasis, the role of platelets in inflammation, allergy and hypersensitivity reactions has recently been recognized. Platelets are metabolically active and contain a full apoptotic system, which make them a good model to study drug toxicity in vitro. Furthermore, they do not proliferate which adds another advantage to the use of platelets as a cell model to evaluate the degree of cell death. Cell proliferation may mask part of cell death in the PBMCs model. Platelets from hypersensitive patients respond to in vitrochemical insult in a similar fashion to PBMCs and the degree of cell death is higher and easier to detect. We also attributed this phenomenon to the lower capacity of platelets for detoxication of reactive metabolites.
In a validation study of the in vitro plasma toxicity assay (iPTA) using rigorous inclusion criteria of identified IDRs cases to sulfa drugs, there was 85% agreement (11 out of 13) between the LTA and the iPTA results in the 13 cases we tested. In the two clinically confirmed cases where the two tests did not agree the LTA was negative and the iPTA was positive. This disagreement between the LTA and the iPTA is probably due to the higher sensitivity of the platelet test to detect patient susceptibility.
In conclusion, the iPTA offers a simplified procedure for in vitro toxicity testing for IDRs with higher sensitivity than the LTA. We believe that the iPTA is more suitable as a diagnostic procedure for IDRs for wider clinical use.